Contamination Control in Ultrapure Chemicals and Water
How small are the particles that our filters are removing?
An average of 4 million cubic feet of water flow over Niagara Falls every minute. Filtration down to 1 ppq is the equivalent of finding and removing one drop of impurity from this flow over 1 day.
FAQ Series
Fundamentals of Contamination Control
Controlling contamination has a clear objective. Improving manufacturing yield by reducing wafer defect rates related to chemical contaminants. Particles, gels, and metals all cause varying types of surface abnormalities that lead to defective products. Liquid filters and purifiers provide a line of defense to prevent defect-causing contaminants from reaching the wafers and substrates. Not all contaminants can be removed, however. Take a look at the most frequently asked questions for the solutions that will help you gain a stronger understanding of the fundamental concepts, mechanisms and best practices to improve your chemical and water filtration and purification processes.
-
Q.) What is the critical function of a filter membrane in semiconductor manufacturing?
A.) A membrane is a selective barrier material that permits certain desirable elements in a fluid stream to pass through while retaining others than that can be damaging to the process. Membranes used in chemical filtration are constructed of polymers and reduce the number of defects on the wafers or substrates by removing contaminants.
-
Q.) Which contaminants are typically removed from chemical processes in semiconductor manufacturing?

- CMP slurries - Particles and gels
- Photochemicals - Particles, metals and gels
- Aggressive acids and bases - Particles and metals
- Dilute acids and base chemistries s- Particles and metals
- Solvents - Metals
-
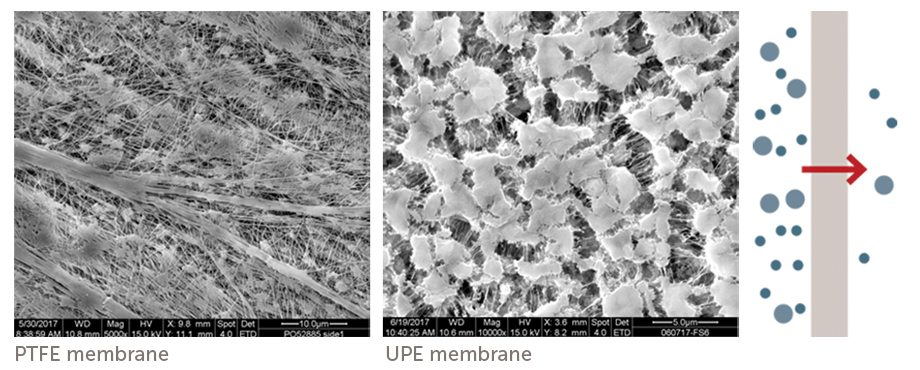
Q.) What are the two most commonly used filter types used in semiconductor manufacturing? How are they different?
A.) Membrane and Depth Filters.
Membrane-based filters utilize a sheet, or flat membrane material and are most commonly used in acids, bases, solvents, photoresists and water. Depth filters utilize a porous polymer block and are most commonly used with CMP slurries. The choice of each type is most commonly determined by the chemical type.
-
Q.) What is the relationship between a filter’s retention rating and its particle size?
A.) As the filter’s retention rating decreases numerically, contaminants are removed in increasingly smaller sizes. As a process becomes more sensitive to smaller particles, a filter with a lower retention rating is required. For example, a 10 nm filter will remove smaller contaminants than the same style with a 20 nm retention rating.
-
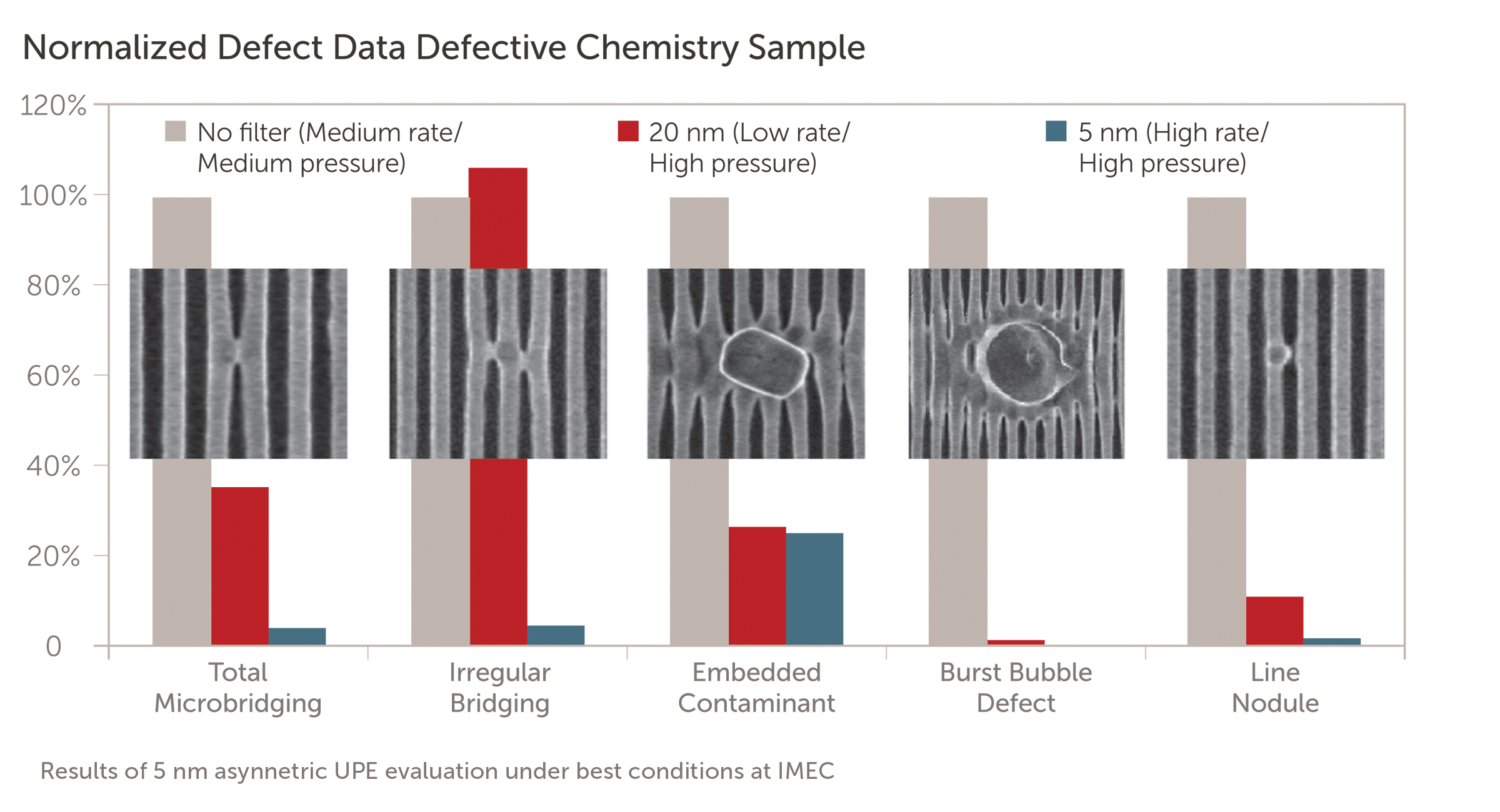
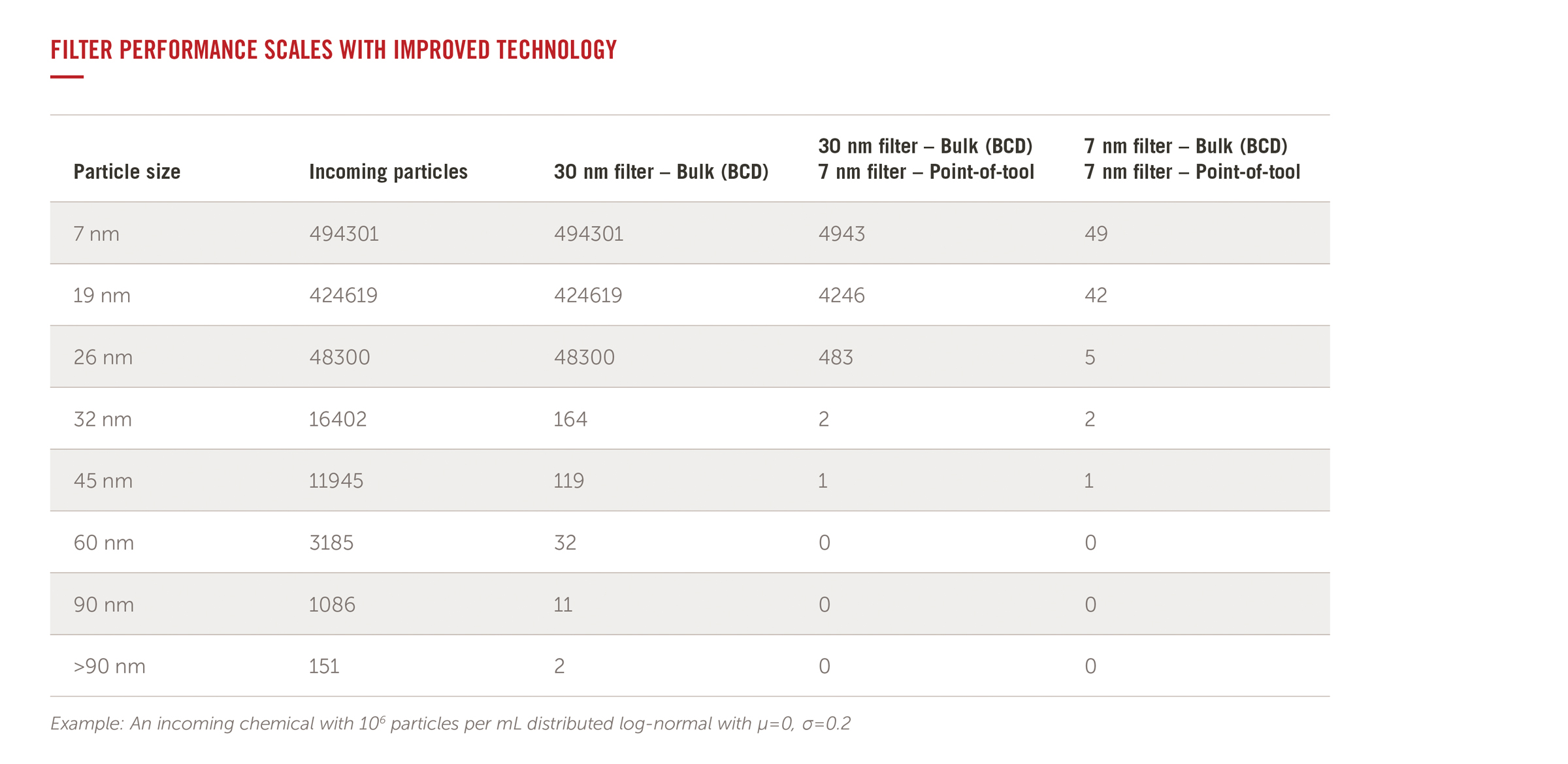
Q.) What is the performance difference between filters?
A.) Every chemical has a concentration of contaminants that should be removed to prevent the formation defects on the wafers. These particles are of various size and every process has a tolerance set for the maximum size allowable.
The chart shown demonstrates examples of how to remove particles of various sizes and concentrations with different filter combinations at the bulk (BCD) and point of tool (POT) filtration points. As the performance of the filters improves (lower retention rating and strategic placement/combination), the particle counts drop to within the tolerance limit.
-
Q.) What influence does a membrane material have on a reducing wafer defects?
A.) Each membrane material has specialized compatibility with each chemistry, but the filter's ability to reduce defects is also dependent upon structure. The physical structure will determine the retention of various sized particles through a "sieving" mechanism.
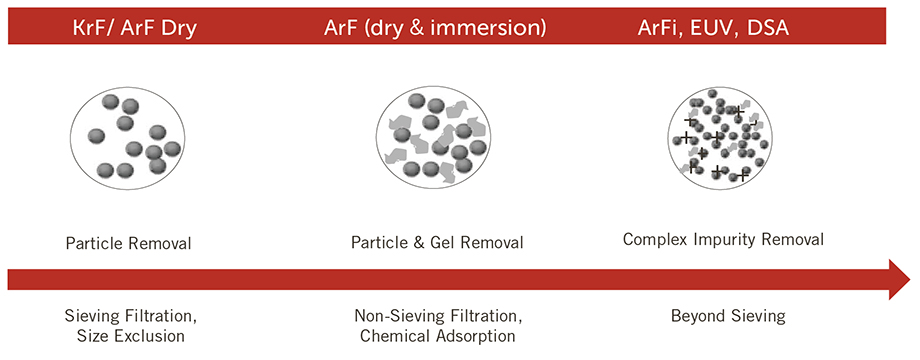
-
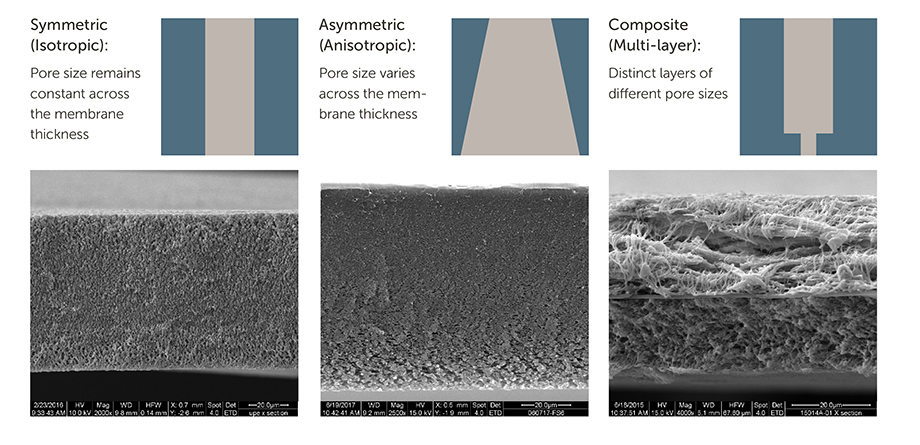
Q.) What are the differences between filter membrane structures?
- Symmetric (Isotropic), pore size remains constant across membrane
- Asymmetric (anisotropic), pore size varies across membrane
- Composite (multi-layer), there are distinct layers of different pore sizes and materials
Each structure is designed for particular performance characteristics found incontamination control programs.
-
Q.) What influences a filter’s retention rating and flow rate?
A) Membrane performance is dependent upon:
- Materials to determine the surface energy of the membrane (wetting ability)
- Device structure to determine the flow path of the fluid (see video)
- Surface area of the membrane inside the filter (flow rate)
- Cleaning techniques to determine the presence of extractables (extractables)
Each of these factors are interdependent and have an influence on the filter's performance.
-
Q.) What are the most important aspects of replacing a cartridge filter in a housing?
A.) Several aspects will influence the performance of the new filter (see video)
- Using the correct PPE (personal protective equipment) - avoids interaction with chemistry
- Proper fit - choosing a compatible design for the housing style
- Material compatibility - choosing a filter that is compatible with the chemistry
- Clean tools and PPE - avoids introducing new contaminants
-
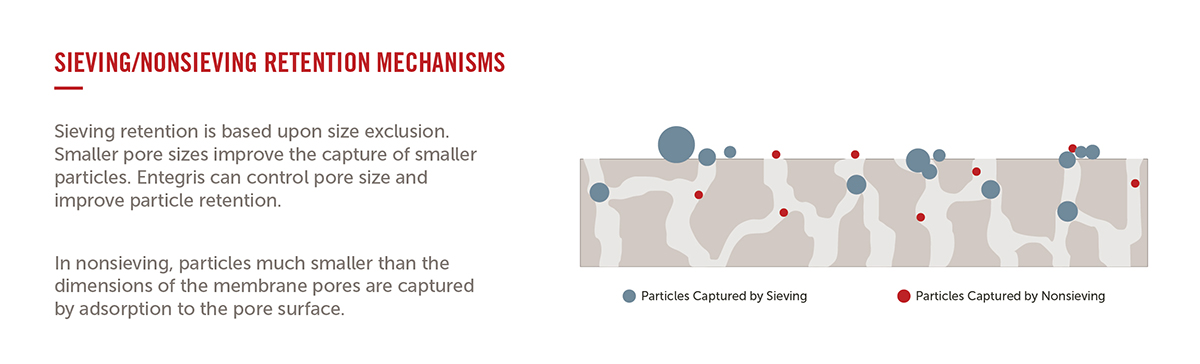
Q.) What's the difference between sieving versus non sieving contaminant removal mechanisms?
A.) Sieving retention is based upon size exclusion.
The physical size of the membrane pore structure will retain the larger sized particles. A smaller pore size structure increases the retention of smaller particles.
Nonsieving retention is based on chemical attraction between the membrane and the contaminant. The particles are typically much smaller than the dimensions of the membrane pores and are captured by adsorption to the filter's membrane surface.
-
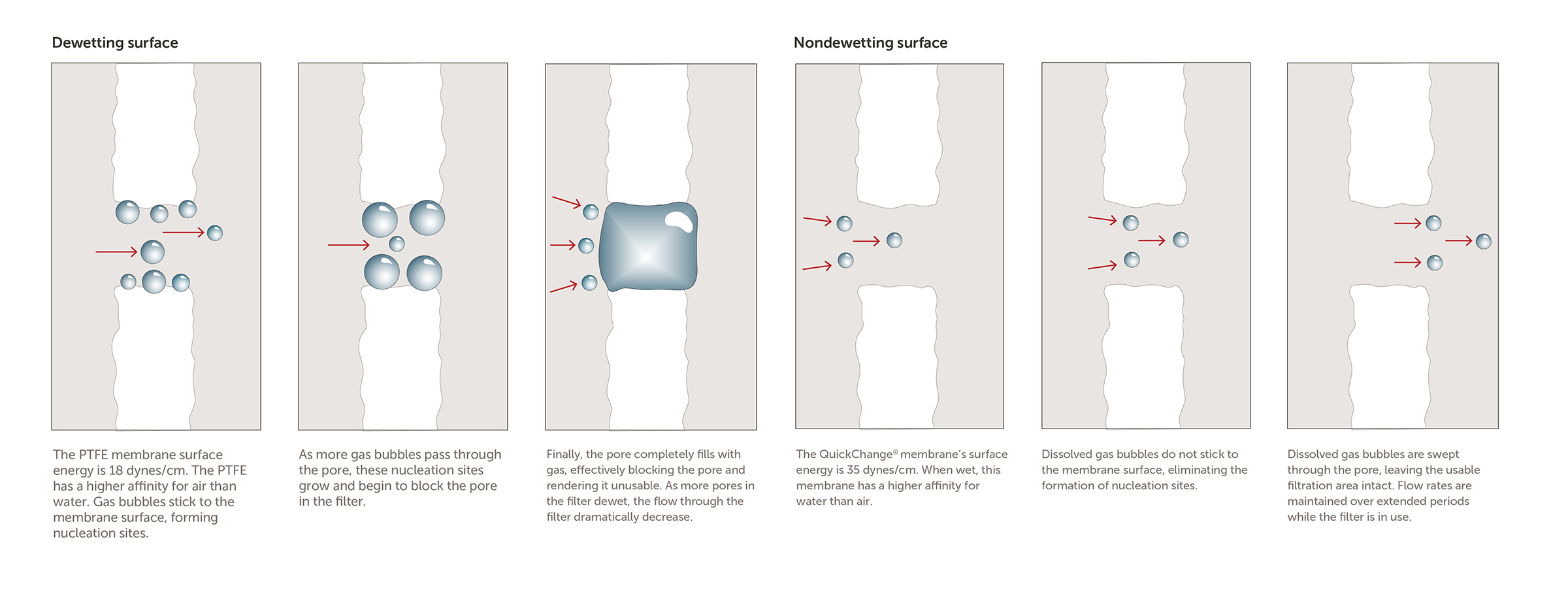
Q.) What is the difference between "dewetting" and a "non-dewetting" membranes?
A.) The term "dewetting" refers to a membranes ability to adsorb a liquid in the open space in the pore structure as a method of preparing it to work effectively.
When a filter "dewets", the fluids in the pore structure release and dry out essentially. This leaves the filter in a less than optimal state of performance.
The mechanism to keep the filter "wet" are surface energies of the chemistry and filter. These both must be in alignment to prevent nucleation sites from forming on the membrane. When this happens, the open spaces in the filters pore structure close and prevent the chemistry from flowing through effectively. For this reason, careful consideration of the chemistry and membrane combinations chosen is an important consideration for optimal performance.
-
Q.) What is the value of a "prewet" membrane relative to the filter?
A.) A prewet membrane is shipped in a fluid solution to enable it to be installed quickly, reducing the tool downtime. Membranes that are not prewet require additional "priming" time and chemical use.
In both types, the goal of the preparation is to "wet" or "wet out" the membrane so that the chemistry will effectively flow through the membrane pore structure and capture contaminants more quickly. If this state is not reached, the chemistry will not flow through effectively and contaminants will not be removed efficiently.
-
Q.) How does a chemical’s surface tension affect membrane compatibility with different chemistries?
A.) Differences between surface tension of a liquid and critical surface energy of a membrane help determine compatibility.
If the surface tension of the fluid is greater than the surface energy of the membrane polymer, the membrane will not automatically wet out.
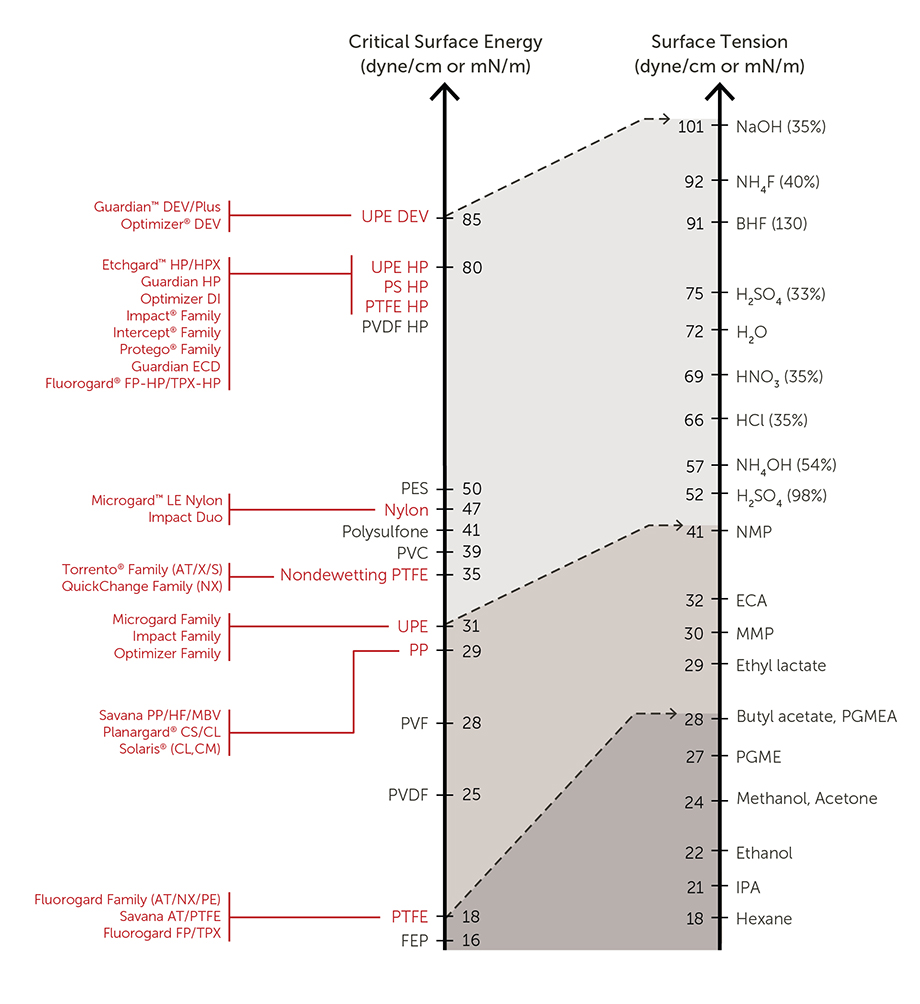
-
Q.) What influences a filter’s retention rating and flow rate?
A.) Membrane performance is dependent upon:
- Materials to determine the surface energy of the membrane
- Device structure to determine the flow path of the fluid (see video)
- Surface area of the membrane inside the filter
- Cleaning techniques to determine the presence of extractables
Each of these factors are interdependent and have an influence on the filter's retention and flow rate performance.
-
Q.) What influence does surface area have on a filter’s flow rate performance?
A.) The membrane surface area of a filter has a direct correlation to the flow rate performance. The higher surface area filters generally provide a higher flow rate. The pleat design of the filter also has an influence on the flow rate performance.
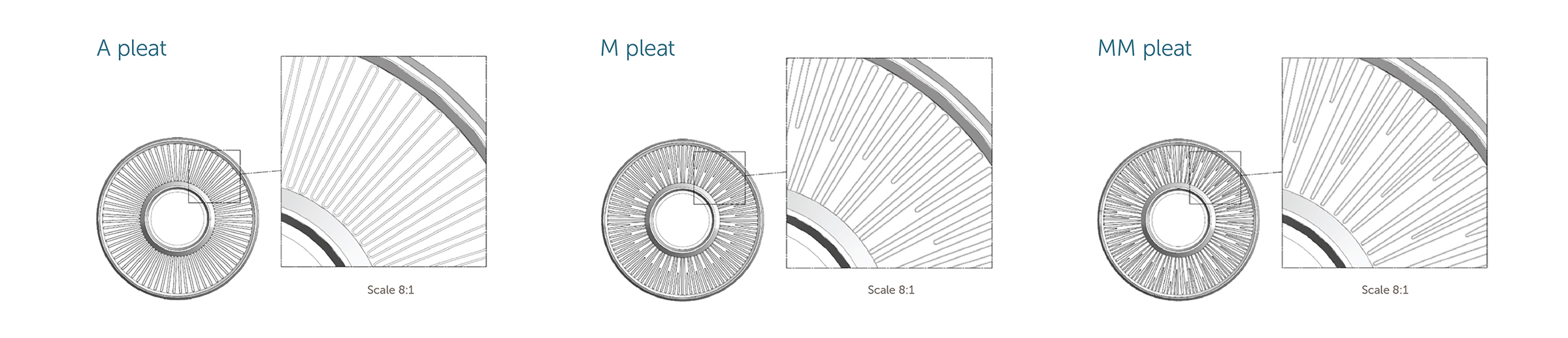
-
Q.) What effect does temperature have upon chemical and o-ring compatibility?
A.) O-rings are a component in a polymer filter designed for use with specific chemical types and under certain conditions.
When an o-ring is installed into a chemical process that is outside its compatibility, it may break down and shed particles into the chemistry. Temperature is also a consideration when choosing an o-ring for a particular process condition. An elevated temperature can further strengthen the reaction between an o-ring and chemistry.
-
Q.) What are the benefits of serial filtration?
A.) Serial filtration utilizes several filters at distinct points in the chemical journey.
This approach provides faster tank cleanup in BCDS (bulk chemical delivery systems) with a progressive increase in the contaminant control performance as the fluids get closer to the water, and an extension of filter lifetime on tool.
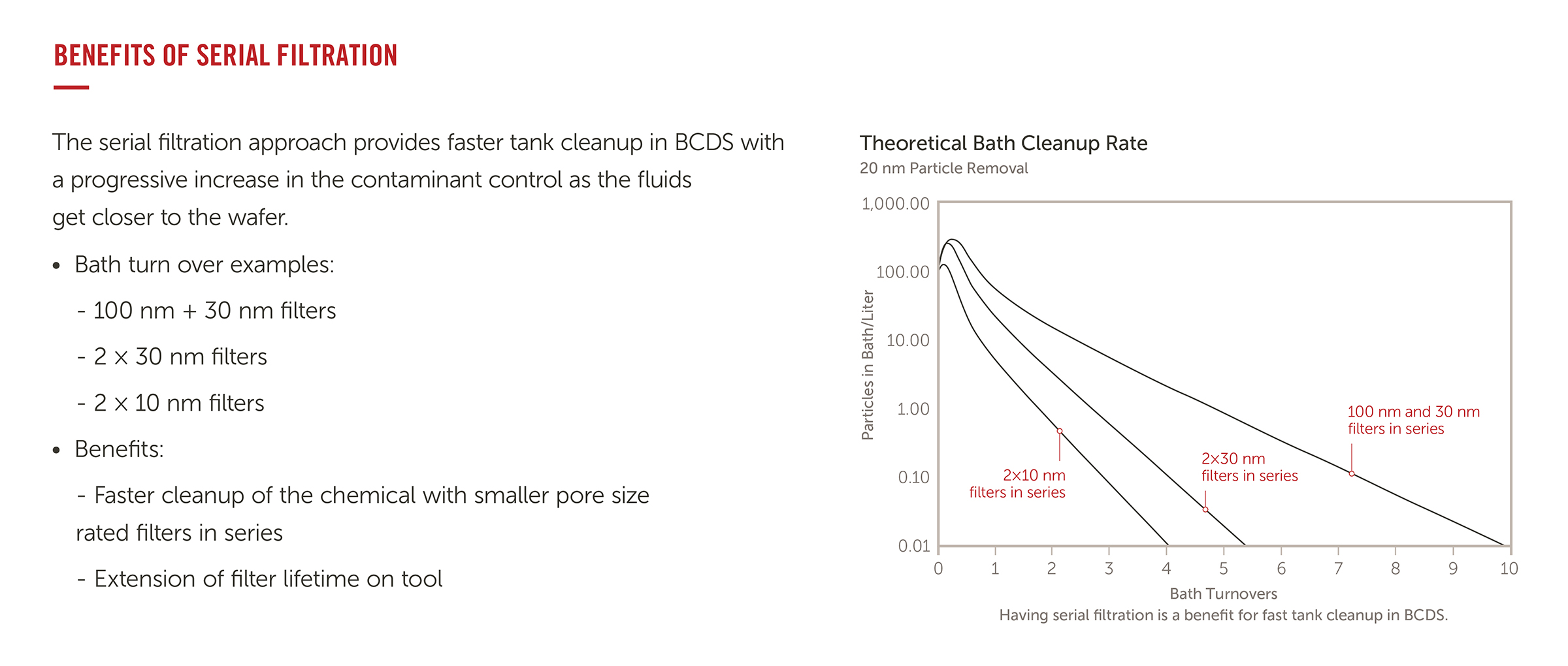
-
Q.) What is the difference between a filter and purifier?
A) A filter is focused on removing particles and gels typically with a sieving removal mechanism (size exclusion) while purifiers are typically used to remove metal ions using nonsieving mechanism (adsorption).
A purifier is a higher level of contamination control in chemical and water purification. While the exterior filter/purifier shell may be the same, the internal membrane is the key difference between the two types.
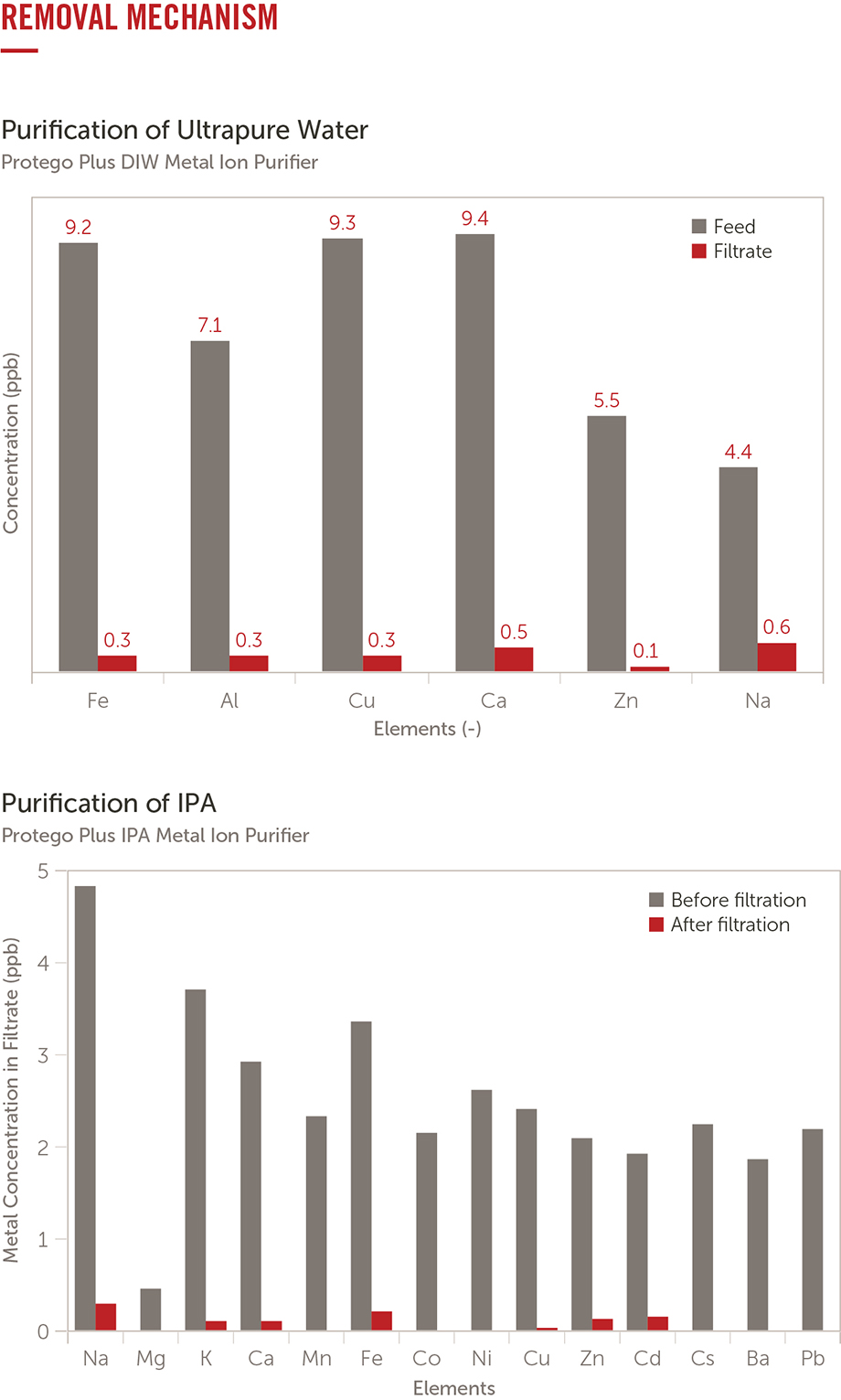
-
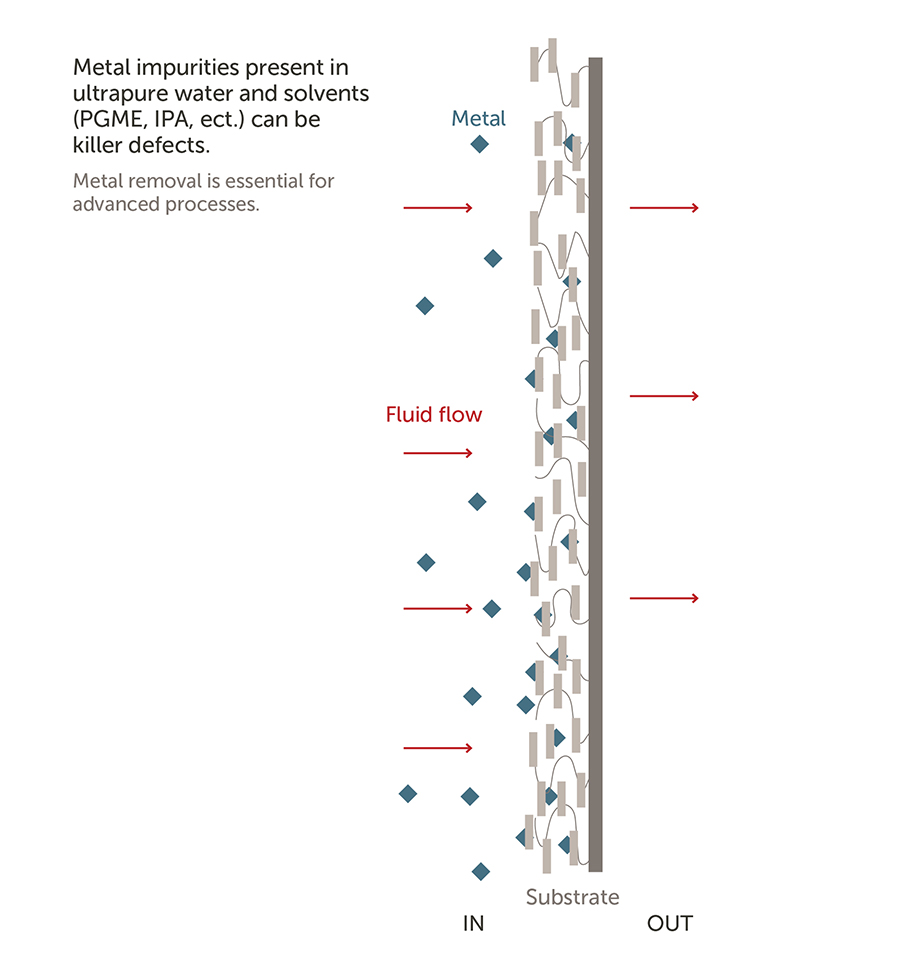
Q.) What is the importance of purification in ultraclean fabrication environments?
A.) Purifiers provide an advanced level of protection from the defect-causing materials found in DI water and solvents using nonsieving removal mechanisms.
-
Q.) Is removal efficiency of metals affected by an increase in flow rate with organic solvents?
The removal of sodium and potassium metal ions from both PMGE and an aqueous solution using a solvent purifier.
Removal of sodium and potassium from an aqueous solution is possible even at different flow rates. In PGME solvent, potassium is effectively removed. However the removal of sodium in PGME solvent is reduced as the flow rate increases. This indicates that in PGME solvent, metals exist not only as dissociated metal ions but also as metal salts. This must be taken into consideration when purification of metals from organic solvents is implemented.
For more details, see the Application Note.